Protocol for determining fertility while breastfeeding and not in cycles
A protocol was developed and evaluated for nonovulating breastfeeding women to determine potential fertility with an electronic hormonal fertility monitor. The amount of required abstinence (i.e., days of potential fertility) through the first menstrual cycle indicated by the fertility monitor was significantly lower (17% of the total days) compared with the amount of abstinence (50% of the total days) indicated by the self-observation of cervical mucus.
Determining fertility with natural biological markers during breastfeeding can be difficult. Breastfeeding not only affects the regularity of cycles (both the length and phases of the cycle), but also common natural indicators of fertility such as cervical mucus patterns and basal body temperature. The transition from breastfeeding not in cycles to being in cycles and to the early cycles after menses returns is often a time when women wishing to use natural methods to avoid pregnancy become pregnant. Current methods of natural family planning (NFP; both temperature-and mucus-based methods) estimate the fertile time during breastfeeding while not in cycles by having women users determine a consistent pattern of cervical mucus or of dryness. Fertility is assumed when there is any change from the consistent pattern and 3 full days after. Waiting for the basal body temperature shift as an indicator of fertility is not too helpful since it is a postovulatory event.
Use of current methods of NFP (during breastfeeding) results in overestimating the actual days of fertility, prolonged and confusing mucus patterns, long periods of abstinence, and an increased pregnancy rate (1-4). Finding more accurate and simple ways of determining fertility while breastfeeding would be a help to couples wishing to use natural methods. The purpose of this pilot study was to evaluate a simple protocol for breastfeeding women who are not cycling and who wish to use a natural method for avoiding pregnancy. The protocol involves the use of an electronic hormonal fertility monitor. The protocol was evaluated by comparing the number of days of required abstinence using the fertility monitor with the self-observation of cervical mucus by breastfeeding women.
This was a retrospective evaluation of fertility charts from 10 breastfeeding women who presented to one of two private university-based fertility education clinics for the purpose of learning how to avoid pregnancy with a natural method. These 10 women eventually were part of a larger study on the effectiveness of a fertility monitor to aid in learning and using NFP. The 10 women participants had a mean age of 33.1 (SD = 6.0; range, 26 -42) and 3.4 children (SD = 1.7; range, 1-7). These women were taught how to track their fertility by observing their cervical mucus and by using a Clearblue Easy Fertility Monitor.
Participants self-observed for cervical mucus every time they voided and before bedtime. Observation of mucus included wiping the vulva with tissue and finger testing at eye level. Cervical mucus was rated by participants on a scale of 1-8, with 1 = dry sensation, no mucus; 2 = not dry, not slippery; 3 = yellow or white, minimal; 4 = yellow or white, sticky; 5 = cloudy, becoming clearer, sticky; 6 = thinner, more stretchy, sticky; 7 = clear, stretchy, and/or slippery; and 8 = slippery, variable amount. Any mucus given a rating of 3 or more was considered the fertile phase and counted as a fertile day.
The Clearblue monitor is a hand-held electronic fertility device designed to identify the fertile phase by detecting a threshold level of estrone-3-glucuronide (E3G) and LH on antibody-impregnated "wick type" test sticks (5-7). The monitor provides either a "low," "high," or "peak" fertility reading. The high reading indicates that a threshold level of E3G has been reached. The peak reading registers when it detects a threshold level of LH. The monitor is designed to detect the optimal fertile days to achieve a pregnancy.
Since the monitor is designed for women who are ovulating and "in cycles," we devised a way of using the monitor to track "artificial cycles." The protocol is as follows:
- Trigger a cycle by pushing the "M" button on the monitor.
- Fast-forward the monitor to day 5.
- The monitor will ask for a test for the next 20 days.
- Test your first morning urine every other day.
- When a high is recorded test the urine every day.
- Retrigger the monitor and fast-forward every 20 days.
- Continue steps 1-6 until you detect a peak reading and resume menses.
- To avoid pregnancy, avoid intercourse on high and peak days and 3 full days after the last peak day.
The participants used a fertility chart to record their cervical mucus observations, the results from the fertility monitor, and acts of intercourse on a daily basis until presumed ovulation and regular cycling returned. Institutional Review Board approval was obtained from the University Office of Research Compliance. Exempt status was granted because there was no identifying information taken from the menstrual cycle charts. The current report is a retrospective fertility chart analysis of the protocol. Descriptive statistics (means, SDs, and percents) and paired Student t-tests were used to determine differences in the estimated time in fertility (i.e., days of abstinence) between the fertility monitor and cervical mucus observations.
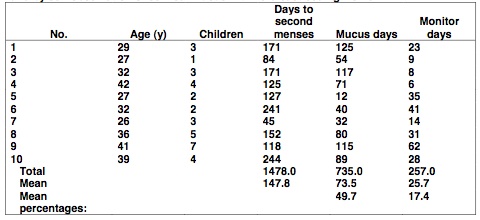
The mean number of days from the first day of charting fertility through the first full menstrual cycle after the first menses was 147.8 (SD = 62.8; range, 45-244) or a grand total of 1,478 days. The mean number of high and peak days on the monitor (i.e., estimated days of fertility) was 25.7 days (SD = 17.6; range, 6 - 62) or 17.4% (257 days) of the total. The mean number of cervical mucus days (i.e., mucus days that indicated fertility) was 73.5 days, (SD = 38.8; range, 12-125 days) or 49.7% (735 days) of the total. There was a statistically significant difference between the mean number of abstinence days as indicated by mucus versus the monitor (t = 3.646; P<.01; see Table 1).
Although this was a small sample, the results do indicate the potential of significantly reducing the required abstinence considerably for breastfeeding women not in cycles with the use of an electronic hormonal fertility monitor. Of interest, the mean number of cervical mucus days that required abstinence with the 10 participants (73.5 days) was similar to the mean of 77 days found in a recent study that investigated the usability of two simplified methods of fertility monitoring during breastfeeding (8).
The protocol described is theoretically based on the understanding that the monitor is designed to detect fairly high levels of E3G (presumably from a mature follicle) and a urinary LH surge before the first ovulation. The protocol involves creating artificial 20-day cycles with the monitor and continuing with the artificial cycles until the first LH is detected. After the LH surge is detected and menstrual cycles resume, the monitor is then used in the normal fashion recommended by the manufacturer.
One disadvantage of the protocol is the expense of the monitor (approximately $200) and the test strips (about $50 for a pack of 30). The cost of test strips average out to be about $25 per month. The monitor, however, can continue to be used as an aid to monitoring fertility and to avoid pregnancy once cycles resume. The average cost of using the monitor during normal cycles will decrease to about $18 per cycle.
Another disadvantage of the monitor is that once it detects a high reading it will continue with high readings (and ask for a test) until it detects an LH surge. This can result in one or two 20-day periods of abstinence for the user of the protocol. To keep costs down, we recommend that users do "every other day" testing (or every third day) until a high reading is detected. Women who are fully breastfeeding and within 6 months of the birth of their child have the option of using the monitor or not and following the lactational amenorrhea method protocol (9). The fertility monitor protocol is initiated when breastfeeding de-2. Labbok MH, Stallings RY, Shah F, Perez A, Klaus H, Jacobson M, et al. Ovulation method use during breastfeeding: is there increased risk of creases and supplements are added to the infantfs diet.
This protocols needs to be tested with a larger sample of couples, for both effectiveness in avoiding pregnancy and the number of required days of abstinence. However, it is clear that the use of this protocol with the fertility monitor provides women with a simple and objective method of determining fertility while breastfeeding and not in cycles.
Richard J. Fehring, D.N.Sc.(a)
Mary Lee Barron, M.S.N.(b)
Mary Schneider, M.S.N.(a)
(a) Marquette University College of Nursing Institute for Natural Family Planning, Milwaukee, Wisconsin; and (b) Saint Louis University School of Nursing, St. Louis, Missouri
Notes
- Kennedy KI, Gross BA, Parenteau-Carreau S, Flynn AM, Brown JB, Visness CM. Breastfeeding and the symptothermal method. Stud Fam Plann 1995;26:107-15.
- Labbok MH, Stallings RY, Shah F, Perez A, Klaus H, Jacobson M, et al. Ovulation method use during breastfeeding: is there increased risk of unplanned pregnancy? Am J Obstet Gynecol 1991;165(Suppl):2031-6.
- Zinaman M, Stevenson W. Efficacy of the symptothermal method of natural family planning in lactating women after the return of menses. Am J Obstet Gynecol 1991;165(Suppl):2037-9.
- Tomaselli GA, Guida M, Palomba S, Barbato M, Nappi C. Using complete breast-feeding and lactational amenorrhea as birth spacing methods. Contraception 2000;61:253-7.
- May K. Home monitoring with the ClearPlan Easy Fertility Monitor for fertility awareness. J Internat Med Res 2001;29(Suppl 1):14A-20A.
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, et al. Prediction of ovulation by urinary hormone measurements with the home use Clearplan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478-82.
- Fehring R, Raviele K, Schneider M. A comparison of the fertile phase as determined by the Clearplan Easy Fertility MonitorTM and self assessment of cervical mucus. Contraception 2004;64:7-14.
- Arevalo M, Jennings V, Sinai I. Application of simple fertility awareness- based methods of family planning to breastfeeding women. Fertil Steril 2003;80:1241- 8.
- World Health Organization (WHO) Task Force. The World Health Organization multinational study of breast-feeding and lactational amenorrhea. III. Pregnancy during breast-feeding. Fertil Steril 1999;72:431-9.
Appendix
Table 1: The number of days of abstinence as indicated by an electronic hormonal fertility monitor and by self-observation of cervical mucus with 10 breastfeeding women.
Article copyrights are held solely by author.
[ Japan-Lifeissues.net ] [ OMI Japan/Korea ]