Breast Cancer and Early Oral Contraceptive Use
"Perhaps the greatest interest and controversy in breast cancer epidemiology has surrounded the questions of whether use of oral contraceptives and estrogen replacement therapy affect the risk for breast cancer." [1, p.298]
More than 10 million women in the U.S. currently use oral contraceptive pills (OCPs)2 and they are used by more than 50 million women worldwide annually. In addition, women are using OCPs earlier in their reproductive lives and for longer periods of time. Obviously, if women who take OCPs -- especially those who take them before their first full-term pregnancy (FFTP) -- are at risk, it has personal as well as worldwide repercussions. The focus of this chapter will be to answer one specific question, namely:
Is the woman who takes oral contraceptive pills at an early age at increased risk for developing breast cancer?
First, we explore some of the background of the oral contraceptive pill and the history of the OCP/breast cancer debate.
Q-8A. What is an OCP and how does it work?
[A more detailed discussion of what an OCP is and how it works can be found in Appendix 5]. (Appendix 5)
A woman's menstrual cycle is regulated by a small gland in the brain called the pituitary gland. It secretes a number of hormones which include FSH (Follicle Stimu rating Hormone) and LH (Luteinizing Hormone), both of which are necessary for ovulation to occur and both stimulate a woman's ovaries to produce two important sexual hormones called progesterone and estradiol-17B.
In the 1920s and 1930s, scientists found methods of obtaining both progesterone and estradiol from animals and discovered their chemical composition. It was soon noted that if either of these compounds were given in high doses, they could inhibit ovulation. Basically, these hormones, when given in high doses, had the effect of "shutting down the pituitary gland" so that the levels of FSH and LH would decrease, thereby inhibiting ovulation.
Researchers soon discovered how to make artificial progestins and estrogens in the laboratory. In the 1950s, it was discovered that the combination of an artificial estrogen and progestin would inhibit ovulation. These were the first OCPs.
Today's OCPs are still made with a combination of an artificial estrogen and progestin. The estrogen is either ethinyl estradiol or mestranol. The body breaks down mestranol into ethinyl estradiol so that the two have very similar effects. The other part of today's OCPs is composed of an artificial progestin. Today's progestins are far more powerful than the body's natural hormone, progesterone. Estranes are the first type of artificial progestin and are about 5 to 10 times more potent than progesterone. They include norethindrone and norethynodrel. A second even more powerful type of progestin are the gonanes, which include norgestrel, gestodene and norgestimate.
Over the years, OCPs have changed. In the 1960s, OCPs were available in sequential or combination form. Sequential OCPs contained an estrogen for 4 weeks of the cycle and added a progestin in the 4th week. They were removed from the market in the 1970s because they increased the incidence of uterine cancer. Combination OCPs contain a fixed amount of an estrogen and progestin, whereas triphasic OCPs, the third type of OCP (also called phasic pills), alternate the ratio of the estrogen and progestin throughout the cycle. Both of these types of OCPs are commonly used today although the use of triphasics has increased over the last 15 years.
How do OCPs work? The combination of these artificial estrogens and progestins function to prevent or stop pregnancy in three ways. First, they inhibit ovulation by inhibiting the pituitary gland and thus FSH and LH. Today's OCPs, which have lower amounts of hormones than the original OCPs, do not totally shut down ovulation. It is estimated that breakthrough ovulation occurs at least 5 to 10% of the time and this is probably a low estimate because some of today's OCPs have estrogen contents as low as 20 micrograms. Second, they may thicken the cervical mucus, making it somewhat more difficult for sperm to travel through. Third, they change the lining of the uterus and often cause an early abortion in those instances when ovulation and conception have occurred. Both pro-life and proabortion groups admit to this effect, with the latter doing so publicly in testimony before the Supreme Court in 19893.
(For a detailed discussion of this as well as an examination of the OCPs mechanism of action, see Appendix 5).
Q-8B: Did scientists study the OCPs effect on animals?
Yes, concerns were raised in 1972 when it~was noted that an OCP containing mestranol and norethynodrel appeared to cause a case of metastatic breast cancer in a female rhesus monkey4. This was especially worrisome because rhesus monkeys rarely develop breast cancer. Until that time only three cases of breast cancer in rhesus monkeys were known. Although it was argued that this was simply a "chance finding," concern grew when it was noted that both beagles5, 6 and rodents7, 8, 9 developed breast cancer when exposed to the hormones contained in today's OCPs.
Q-8C: What effect does OCP use have on human breast cells?
In 1989, Anderson et al10 published a classic paper regarding the influence of OCP use on the rate of breast cell division. They found that nulliparous women who took OCPs had a significantly higher rate of breast cell division (measured via thymidine labeling index) than nulliparous women who did not take them. The difference in parous women (ie, women who have had at least one child) who either did or did not take OCPs was not as significant. This is illustrated in Figure 8A where nulliparous women who were either taking or not taking OCPs (ie, labeled "on," or "off") were compared to parous women who were "on" or "off" the Pill.
Figure 8A: Rate of Breast CELk Division in Women Who Take the Pill (adapted from data from Anderson et al) [10]
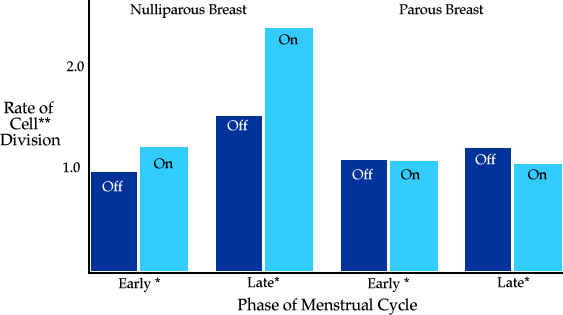
[*Early or late phase of the secretory portion of a woman's menstrual cycle.]
[**Rate of cell division was measured by TLT (thymidine labeling index).]
This was especially significant because it is known that, in general, cells which have an increased rate of cell division are more likely to become cancerous. Another study by Williams et al11 found that women who took OCPs had a marked reduction of estrogen receptor positive breast cancer cells when their cells were examined (ie, estrogen receptor positive breast cells respond much better to treatment than estrogen receptor negative breast cancer cells). Additional information as to specifically how either estrogen or progestin could cause breast cancer is given in the addendum at the end of this chapter.
Q-8D: Does the fact that OCP use causes early abortions explain why it increases the risk of breast cancer in young women?
Daling12 and Rookus13 have noted that abortions prior to 8 weeks of pregnancy result in an increased risk of breast cancer, but do the very early abortions from use of hormonal contraceptives such as the OCP and DepoProvera also elevate the risk? Stewart et al have shown that estradiol and progesterone levels already start to rise above baseline levels within 4 days of conception, thus prior to implantation and before hCG levels begin to rise [14, p.1472]. An early abortion would cause a sudden fall in the levels of these hormones. Could these early hormonal fluctuations be playing a role? To this author's knowledge, no one has asked or studied this question. Many hormone levels change in pregnancy and perhaps with advances in technology we may be able to measure the hormone levels in early pregnancy more accurately, such as Stewart et al have. Perhaps in time, a variant of Stewart et al's methods may be able to quantify more precisely how often OCP use causes early pregnancy and abortion, in addition to enabling researchers such as Anderson et al to be able to perform studies on breast cells during the very early stages of pregnancy.
Q-8E. What is the historical evidence from studies of humans that oral contraceptives cause breast cancer when taken by women early in their lives?
In 1981, Pike et al15 noted that women who took OCPs for 4 or more years prior to their first full-term pregnancy (FFTP) experienced a 125% increased risk in breast cancer, whereas women who took them for 8 or more years prior to their FFTP had a 250% increased risk. This startled the research world and led to additional studies, including a very large American study called the CASH trial in the early 1980s (ie, Center for Disease Control's Cancer And Steroid Hormone study). The researchers originally reported in 1983 that: "Oral contraceptive use before a woman's first pregnancy did not increase her risk of breast cancer significantly more than other methods of delaying first pregnancy."16 As time progressed, however, and further "cases" were included in the study group, the CASH study showed some very disturbing results. By 1993 it showed that women under the age of 44 had a 40% increased risk in breast cancer, which was statistically significant in the 35 to 44 year-old age group17.
Later in England, Chilvers et al18 published the results of another large study called the United Kingdom National Study. They showed that young women under the age of 36 who had used oral contraceptives for at least 4 years before their FFTP had at least a 44% increased risk in breast cancer. The last large study was performed in 1995 by Brinton et al19. It showed a 42% increased risk for women who used OCPs for more than 6 months prior to their FFTP.
Q-8F: If the major studies showed the risks that you have just mentioned, then why do doctors fail to inform their patients of those risks?
That is a good question. Part of the problem is that because the OCP/breast cancer debate is complicated, most people have to rely on what "the experts" tell them. Unfortunately, it does not help when major medical journals and medical associations fail to stress the dangers of early oral contraceptive use.
A good example of this occurred recently in the study printed in a condensed version in The Lancet20 and in complete form in Contraception21. This was and remains the largest meta-analysis regarding the studies of OCPs and breast cancer. Researchers from around the world studied and combined data from 54 studies, involving 26 countries and 53,297 women who had breast cancer. It concluded: "Women who are currently using combined oral contraceptives or have used them in the past 10 years are at a slightly increased risk of having breast cancer diagnosed, although the additional cancers tend to be localized to the breast. There is no evidence of an increase in the risk of having breast cancer diagnosed 10 or more years after cessation of use..." Unfortunately, this study is known more for what it did say, than what it did not say. There were several major problems with the study, which is analyzed in depth in Appendix 5. The largest weakness was the failure to report any evidence of what the pooled risk of oral contraceptive use before a FFTP was in women less than 45 years old. The researchers of the study did examine the risks of women who had used OCPs before age 20, but this certainly is not the equivalent of studying premenopausal women who used OCPs prior to their FFTP. An additional problem is that the Oxford study pooled data from studies which examined women with breast cancer from as far back as the early and mid-1970s [21, p.5S].
Q-8G: Why does the fact that the Oxford study pooled data from early studies affect its results?
The Oxford study pooled the results of studies which interviewed women with breast cancer in the early and mid-1970s [21, p.5S]. Taking data from studies which interviewed women before 1980 will tend to underestimate the risk of developing breast cancer from early OCP use. Why? Data from studies before 1980 would be unlikely to show much risk because the latent period was too short and few women used OCPs for significant periods of time prior to their FFTP in the late 1960s and early 1970s as compared to women of the late 1970s and 1980s [21, p.9S]. Thomas warned of this problem years before the Oxford study was published: "The major limitation of this effort (de, the Oxford study), of course, is that all existing data sets have limited information on use of oral contraceptives by young women for a prolonged period." [22, p.362]
Q-8H: What about the question of 'length of use" before a FFTP; did the Oxford study address that?
No. One of the biggest problems with the Oxford study is that it failed to ask the question of what the risk of OCP use was prior to a FFTP in premenopausal women. Another major weakness is that the study failed to ask the question of what the risk of long-term OCP use (eg, greater than 4 years) before a FFTP was. This is especially important because women in Western countries today are using OCPs for far longer periods of time than women did in the 1960s and early 1970s. We hope that some researchers will perform a re-analysis of the Oxford meta-analysis and will have the courage to ask the question: What is the risk of breast cancer to women who were under the age of 45 as of 1990 and who used OCPs for 4 or more years prior to a FFTP, when confined to the studies done after 1980, or better yet, after 1985?
Q-8I: Has anyone done a mesa-analysis that examined the question of risk to women under the age of 45 who had taken OCPs prior to their FFTP?
Yes. Two different researchers have addressed this question. Thomas et al, in 1991, found that women who took OCPs for extended periods of time prior to their FFTP had a 44% increased risk [RR=1.44 (1.23-1.69)] of developing breast cancer23. In 1990, Romieu pooled studies of women under the age of 45 who had taken OCPs for 4 or more years prior to their FFTP and found that they had a 72% increased risk [RR=1.72 (1.36-2.19)] of developing breast cancer when she restricted her analysis to those studies done after 198024.
Q-8J: What are the largest retrospective studies to date and how does one find them?
This author used Med-Line, which is the name of a computer program that allows the researcher to find major journal articles on virtually any medical subject (it can be accessed through the Internet). In addition, the extensive bibliography found in the Oxford study in The Lancet(20) was used to obtain virtually every major retrospective study that gathered the bulk of its data after 1980 and examined women under the age of 45, in regard to their history of oral contraceptive use.
After gathering all of the retrospective studies, the largest were selected, specifically, those studies that had about 750 or more women under the age of 45. Some studies made no comment on OCP use prior to a FFTP. When this occurred, this author looked for the next closest approximation to the parameter of "early OCP use." A parameter of OCP use within 5 years of menarche was chosen as a close approximation of OCP use prior to a FFTP, if no data on the latter were available. What, then are the largest studies and what do they show?
| Table 8A | |||
|---|---|---|---|
| The Four Large Studies That Measured Early OCP Use and the Risk of Breast Cancer to Women Under the Age of 45 | |||
| Author | Year | Size of Study | Findings |
|
Wingo (17)
CASH Study |
12/80-82 | 2089 less than age 45 | 40% increased risk ages 20-44 |
| Rosenberg25* | 1977-1992 | 1427 less than age 45 | 88% increased risk** |
| White26 | 1983-1990 | 747 less than age 45 | 50% increased risk: for use within 5 years of menarche (parous women) |
| Brinton (19) | 5/90-12/92 | 1648 less than age 45 | 42% increased risk (parous women)** |
| * This study did not control for abortion. | >|||
| ** Calculated from raw data found in their paper—see end of chapter for details. | |||
Q-8K: What do these results mean?
In summary, the four largest retrospective studies of parous women under the age of 45 all show at least a 40% increased risk to women who took OCPs prior to their FFTP or within 5 years of menarche.
Q-8L: Could you explain a little bit about each study?
(This answer is somewhat technical and may be skipped without a loss of continuity).
Certainly. The CASH study was mentioned earlier. It has the largest group of women under the age of 45, specifically, 2089. Because the CASH data were taken from interviews with women during the years of 1980 through 1982, it means that it detected the risk of early contraceptive use and breast cancer despite the fact that the study had a short latent period. The authors admit this themselves: "However, because oral contraceptives have only been marketed in the U.S. since the 1960s, we were unable to study any possible very late effects of their use" [27, p.409]. As this author noted earlier, because women started taking OCPs more frequently before their FFTP in the 1970s, the effect of earlier OCP use and breast cancer would be picked up more strongly in studies which took their data after 1985. In 1993, a Cancer supplement issue (17) reported the following results from the CASH study for women who took OCPs prior to their FFTP:
Women aged 20 to 34 years old had a 1.4 (0.9-2.4) risk and women aged 35 to 44 years old had a 1.4 (1.1-1.8) risk (in other words a 40% increased risk). Unfortunately, the authors did not combine the data for the 20 to 44 year-old age group. Because the 35 to 44 year-old age group had about 10 times as many women in it as the 20 to 34 year-old age group, combining the data would have likely yielded a statistically significant risk of 40% for the combined age group of women aged 20 to 44 years old.
Two other researchers have provided helpful insights into the CASH study. Peto's re-analysis of women aged 20 to 44 years old in the CASH study28 showed a RR of 1.29 (1.03-1.61) in women with 0-3 years of OCP use prior to their FFTP and a RR of 1.48 (1.15-1.91) in women with 4 or more years of OCP use prior to their FFTP. He noted that "whether the results of this and other studies prove a causal relation between OCP use and breast cancer in young women will continue to be debated—but the CASH results, at least for use before first full-term pregnancy, appear to support rather than weigh against the hypothesis." Pike and Bernstein also supported Peto's findings: "The CASH study as reported in women aged 20-44 is thus positive."29
Despite these findings, the lead author of the CASH paper, Phyllis Wingo, wrote in her abstract: "Available data provide no reasons to change prescribing practices or the use of OCP that are related to the breast cancer risk." Why an author would downplay an increased breast cancer risk of 40% to women less than 44 years old is inexplicable. In addition to this, it must be noted that the CASH study also suffered from a significant stack effect, the "death factor," and a fairly short latent period30. Each of these effects would serve to give an even higher risk had they been adjusted for. Therefore, a CASH finding of 40% risk for early OCP use in women less than age 44 is a very conservative estimate.
Rosenberg performed another large study which was published in The American Journal of Epidemiology in 1996. She, like the researchers in the CASH study, failed to combine the data for the 25 to 34 and 35 to 44 year-old age groups [25, p.33]. Combining the data leads one to an 88% increased risk for OCP use prior to a FFTP in women aged 24 to 44 years old (for the calculation of this see the end of this chapter). Rosenberg's increased risk is especially significant in that she had a huge stack effect (20% of "controls" were aged 25 to 34 years old, vs. only 9% of "cases") and a short latent period (ie, some of her data came from the late 1970s) Last, the study was supported indirectly through a grant from Merrell Dow Inc. (which has now merged with Hoechst Inc.) and Hoffman-La Roche Inc., both of whom support the Slone Epidemiology Unit, where Dr. Rosenberg works. All three of these weaknesses would tend to diminish the risk (ie, 88%), making it a very conservative estimate.
White and Daling published the third study in the Journal of the National Cancer Institute in 1994 [26]. They noted a 50% increased RR of 1.50 (1.1-2.2) in parous women who took the OCP within 5 years of menarche and had taken it for at least 1 year. White's study suffered from the stack effect (9% "cases" and 17% of "controls" in the youngest age group, 21 to 30 year olds) as well as a significant death factor. Over 10% of "cases" died or were too ill to be interviewed. In addition, the study limited its focus to only white women, thereby eliminating young black women who are at particular risk from early OCP use (see Chapter 11). All of these factors would have tended to increase the relative risk had the study been adjusted for them.
Brinton's study [19] from the National Cancer Institute (NCI) is the last of the four research projects which studied a large group of women prior to the age of 45. It has the advantage of a long latent period, which makes it especially significant. In Table 5 of her study, Brinton noted the relative risks for women who took OCPs for different lengths of time prior to their FFTP.All of the four subgroups shown had an increased risk of breast cancer, with two of the subgroups showing a statistically significant result. Women who took OCPs between 6 months and 2 years prior to their FFTP had a 40% increased risk [RR= 1.40 (1.1-1.8)], whereas women who took OCPs between 4 and 5 years prior to their FFTP had a 67% increased risk [RR=1.67 (1.2-2.3)]. However, Brinton et al failed to combine the overall data for all of the women who took OCPs for more than 6 months prior to their FFTP in spite of the fact that this is a simple calculation. Combining the data leads to a calculated odds ratio (OR) of 1.42 (de, a 42% increased risk) for women who took the OCP prior to a FFTP -- a result which would have almost certainly been statistically significant had the authors presented it (see end of this chapter for calculations). Perhaps Dr. Brinton could explain why this pivotal calculation was omitted.
Q-8M: Can you comment on why a recent large study published by researchers at Harvard claimed to show no increased risk of developing breast cancer for women who had taken OCPs for 5 years or more prior to their FFTP?
In 1997, a group of researchers at Harvard Medical School led by Dr. Hankinson published a study in Cancer Causes and Control31. It based its conclusions on data taken from the Nurses' Health Study and claimed to show that women who took OCPs for 5 years or more prior to their FFTP had no increased risk of breast cancer com pared to women who never took OCPs [RR=0.7 (0.241.31)]. Unfortunately, the study's design appears to have been badly flawed.
Q-8N: Can you identify the flaws?
The researchers compared women with breast cancer who took OCPs for 5 years or more prior to their FFTP (eg, let's refer to these women as Group A] to women with breast cancer who never took OCPs [eg, Group B).
It is known that women took OCPs for longer periods of time earlier in their reproductive lives in the 1980s and 1990s than in the 1960s and 1970s as was clearly noted in the Oxford study [21, p.9S; Tables 14 and 15]. So any group of women who had taken OCPs for 5 years or more prior to their FFTP (de, Group A) would have been more likely to have done so while in their late teens and 20s in the 1980s or 1990s, whereas women in Group B (who never took OCPs) would be more likely to contain a distribution of women who would have been in their late teens and 20s in either the 1960s, 1970s, 1980s or 1990s. But this strongly supports the contention that women in Group A would have a lower average age and a shorter follow-up time than the women in Group B, which would of course invalidate the study's conclusions.
It is frightening to note that the Harvard team presented NO DATA on either the average age of women in the noted groups or their respective lengths of follow-up time. The research team instead chose to follow the noted groups in "person-years" as their measure of follow-up time. This is the length of a follow-up period derived from the number of women followed, multiplied by the average number of years they were followed. For example, if group A had 100 women who were followed for 10 years, the total amount of follow-up time would be 100 x 10 = 1,000 person-years. But if group A had 250 women who were followed for 4 years it would also have 1,000 person-years of follow-up time. This is totally inadequate because the measure of "person-years" gives no data on the length of follow-up time in actual years, and without this information the study must remain suspect because it was noted that women in group A most likely had both a younger average age and were followed for a shorter period of time than the women in group B.
Q-80: Is there any way that the public will ever have access to the necessary data that was not presented in the Harvard study?
I am not sure. This author tried in vain to obtain the answers to three basic questions over a 6 month period of time from three different researchers involved in the Harvard study. Attempts were made via e-mail, phone calls and certified mail. It is ironic that one cannot obtain access to data from these researchers especially because their study obtained its data from the Nurses' Health Study, a study which is funded by citizen tax dollars through a grant via the National Cancer Institute (NCI). The simple and necessary questions that need to be answered are presented at the end of this chapter. Until the noted researchers at Harvard make their data available for all to see, the study's conclusions must remain suspect.
Q-8P: What should women who take OCPs prior to their FFTP be told?
They should be told that all of the collective data we have point to an estimated increased risk of breast cancer of 40% to women who have used OCPs before their FFTP.
Next Page:
Addendum 8A:
1,
2
Article copyrights are held soley by author.
[ Japan-Lifeissues.net ] [ OMI Japan/Korea ]